Monitoring pulmonary vein stenosis: what modality and how frequently?
Currently, there is no consensus as to what modality and how frequently one should monitor the progression of pulmonary vein stenosis. Data from the PVS registry will try to address this and provide consensus statements.
One of the key objectives it to identify patients with rapidly progressive disease in a time frame which would avoid missing therapeutic windows of opportunity. Slow progressors and fast progressors may require different strategies for surveillance. Data from the PVS Network registry be used to develop criteria for identifying patients who might have a propensity for rapid progression.
In most centres, use of complementary imaging modalities allows for early detection of progressive pulmonary vein stenosis. There is, however, considerable variability between centers in terms of the imaging modality used for surveillance.
One of the key objectives it to identify patients with rapidly progressive disease in a time frame which would avoid missing therapeutic windows of opportunity. Slow progressors and fast progressors may require different strategies for surveillance. Data from the PVS Network registry be used to develop criteria for identifying patients who might have a propensity for rapid progression.
In most centres, use of complementary imaging modalities allows for early detection of progressive pulmonary vein stenosis. There is, however, considerable variability between centers in terms of the imaging modality used for surveillance.
Which modality is best to use for surveillance?
The most common screening modality used to follow children with pulmonary vein stenosis is echocardiography. While it is minimally invasive and does not require sedation, it is important to recognize it is important to recognize the limitations of echocardiography as a screening tool.
Echocardiography is most accurate at detecting stenosis at the veno-atrial junction and when there is balanced circulation between both lungs. As stenosis moves into the intraparenchymal segments, echo may not be able to reliably detect the severity of the stenosis due to inability to image through the lung parenchyma. In addition, flow redistribution from lung segments with obstructed pulmonary veins to unobstructed segments has been detected with magnetic resonance angiography (MRA) flow studies (Greenway J et al, 2011). Consequently, decreased pulmonary venous flow may result in a decrease in echocardiographically-derived pulmonary vein gradients despite progression of stenosis within the involved pulmonary vein. Flow redistribution is an important phenomenon in the context of unilateral pulmonary vein stenosis and other congenital lesions involving the pulmonary artery. Likewise, differential high flow states can overestimate the gradients.
Diagnostic catheterization is an invasive test that can offer diagnostic insights into pulmonary vein diameters throughout the lung and correlative pressure data in throughout the pulmonary vasculature and right ventricle. Importantly, catheterization offers potential for therapeutic interventions with balloon dilations and stent placement. Risks associated with the procedures and the associated anesthetic in the context of right heart failure are important considerations ( Esch JJ et al, 2015).
3D imaging such as CT angiography (CTA) scans and MRA imaging are useful modalities to visualize the pulmonary veins and to allow for objective quantification of cross-sectional area of the pulmonary veins (Hirsch et al, 2006; Grosse-Wortmann L et al 2007; Greenway SC et al, 2011). MRA has the advantage by offering functional measurements, with the flow in the pulmonary veins being quantified. MRA imaging often requires general sedation, although 'feed and sleep' protocol are becoming increasingly commonly utilized to avoid sedation. In areas of the lung where flow redistribution is occurring, the low flow state can make it difficult to appreciate the pulmonary vein patency distal to the area of stenosis.
Lung perfusion scans are another way to quantify perfusion to various lung segments, and can provide complementary information, similar to flow measurements done with MRA (Drubach LA et al, 2015).
Echocardiography is most accurate at detecting stenosis at the veno-atrial junction and when there is balanced circulation between both lungs. As stenosis moves into the intraparenchymal segments, echo may not be able to reliably detect the severity of the stenosis due to inability to image through the lung parenchyma. In addition, flow redistribution from lung segments with obstructed pulmonary veins to unobstructed segments has been detected with magnetic resonance angiography (MRA) flow studies (Greenway J et al, 2011). Consequently, decreased pulmonary venous flow may result in a decrease in echocardiographically-derived pulmonary vein gradients despite progression of stenosis within the involved pulmonary vein. Flow redistribution is an important phenomenon in the context of unilateral pulmonary vein stenosis and other congenital lesions involving the pulmonary artery. Likewise, differential high flow states can overestimate the gradients.
Diagnostic catheterization is an invasive test that can offer diagnostic insights into pulmonary vein diameters throughout the lung and correlative pressure data in throughout the pulmonary vasculature and right ventricle. Importantly, catheterization offers potential for therapeutic interventions with balloon dilations and stent placement. Risks associated with the procedures and the associated anesthetic in the context of right heart failure are important considerations ( Esch JJ et al, 2015).
3D imaging such as CT angiography (CTA) scans and MRA imaging are useful modalities to visualize the pulmonary veins and to allow for objective quantification of cross-sectional area of the pulmonary veins (Hirsch et al, 2006; Grosse-Wortmann L et al 2007; Greenway SC et al, 2011). MRA has the advantage by offering functional measurements, with the flow in the pulmonary veins being quantified. MRA imaging often requires general sedation, although 'feed and sleep' protocol are becoming increasingly commonly utilized to avoid sedation. In areas of the lung where flow redistribution is occurring, the low flow state can make it difficult to appreciate the pulmonary vein patency distal to the area of stenosis.
Lung perfusion scans are another way to quantify perfusion to various lung segments, and can provide complementary information, similar to flow measurements done with MRA (Drubach LA et al, 2015).
What frequency of monitoring is best for surveillance?
The optimal frequency of surveillance imaging is not defined and there is variations amongst individual centres. The balance must be struck between catching the silent progression of the disease and risks associated with repeated sedation and radiation exposure. The frequency of imaging should ultimately be dictated by the velocity of disease in the individual child, the majority of institutions have generic frameworks for surveillance as disease progression is typically silent and to ensure early identification to facilitate early intervention.
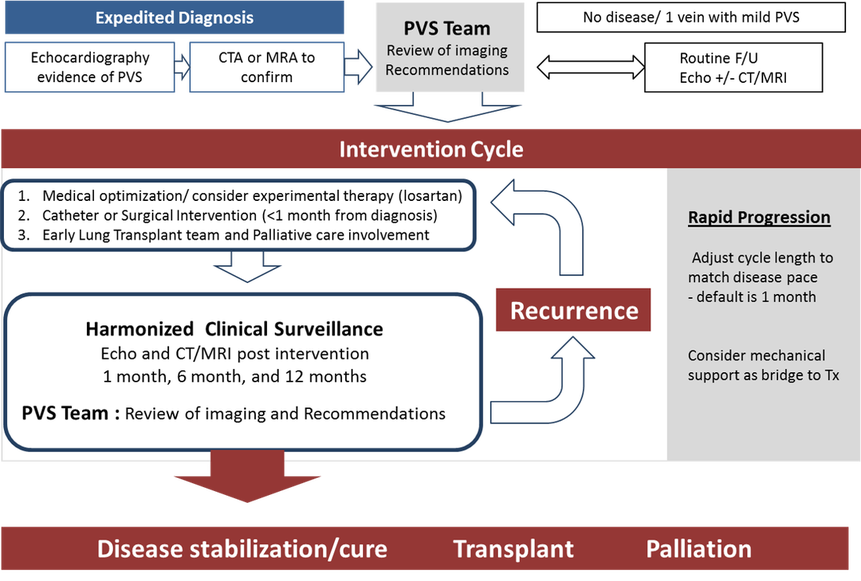
Clinicians can feel isolated and struggle with how best to treat patients with PVS. The relative rare occurrence of PVS, reports of poor prognosis, and the absence of consensus of best practices makes clinical decision-making difficult for children with PVS. Implementation of a dedicated multidisciplinary PVS clinical team comprised of a group of interested clinicians can be utilized to improve care for children with PVS. At our institution, the PVS team meets regularly to review inpatients and outpatients with PVS. Review of surveillance imaging and recommendations for
Clinicians can feel isolated and struggle with how best to treat patients with PVS. The relative rare occurrence of PVS, reports of poor prognosis, and the absence of consensus of best practices makes clinical decision-making difficult for children with PVS. Implementation of a dedicated multidisciplinary PVS clinical team comprised of a group of interested clinicians can be utilized to improve care for children with PVS. At our institution, the PVS team meets regularly to review inpatients and outpatients with PVS. Review of surveillance imaging and recommendations for
How to maximize surveillance and care: Team Approach
Clinicians can feel isolated and struggle with how best to treat patients with PVS. The rare occurrence and the absence of consensus of best practices makes clinical decisions difficult for children with PVS. Implementation of a dedicated PVS clinical team, which is comprised of a group of interested clinicians, can be utilized to improve care for children with PVS. At our institution, the PVS team will meet regularly to review inpatients and outpatients with PVS. Review of surveillance imaging and recommendations for treatment support clinicians treating children with PVS and provide institutional consistency to help evolve treatment practices ( Figure)